Reliability Assurance System
Reliable, safe, and sincere manufacturing under thorough quality assurance
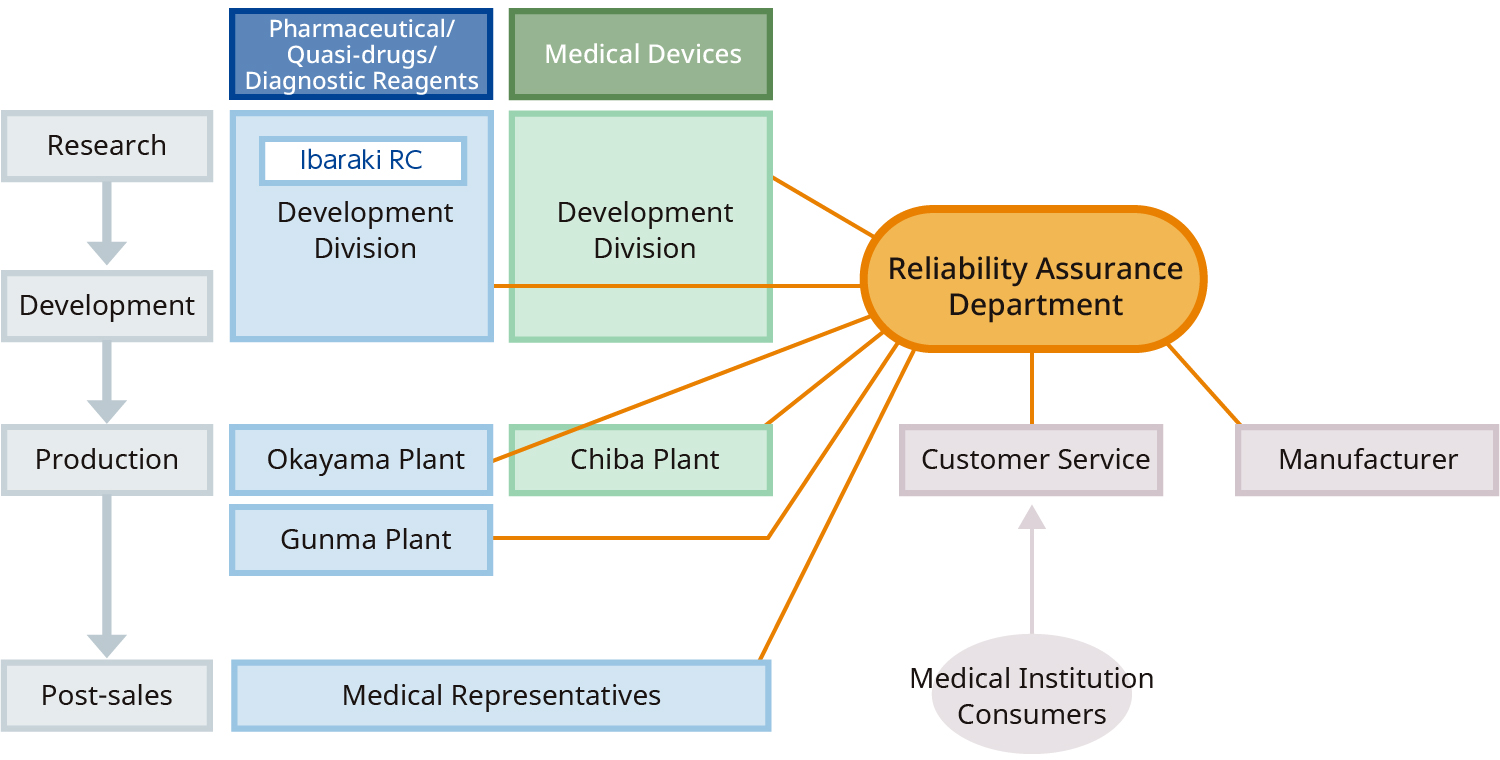
Alfresa Pharma has established and maintains a system in which its R&D, production (Okayama Pharmaceutical Plant, Gunma Plant, Chiba Plant), safety management, and quality assurance departments work in close collaboration to guarantee the reliability of the pharmaceuticals, diagnostics, and medical devices it manufactures and sells throughout the product lifecycle, from research and development to production and post-marketing. In addition to complying with GxP and other reliability standards, we aim to ensure and improve the quality of each of our "people, products, and information" by meeting international standards such as ISO13485 and CE marking.
Quality Assurance Flow